At a Certain Temperature the Equilibrium Constant
The equilibrium constant Keq for the reaction. If 0300 mol of SO3 and 0300 mol NO were placed in a 200 L container and allowed to react.
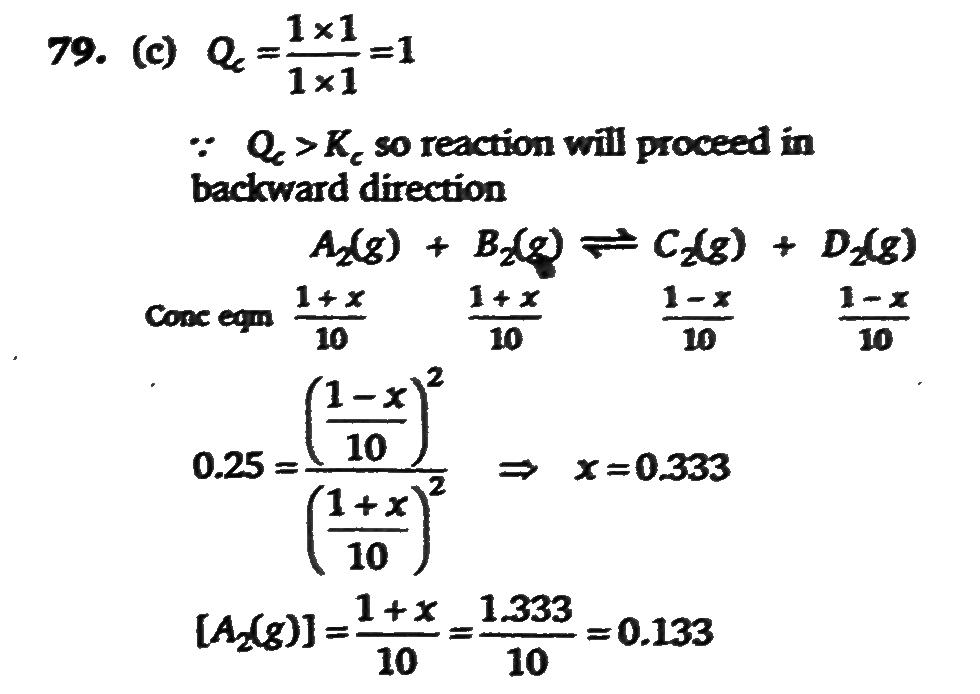
At A Certain Temperature The Equilibrium Constant Kc Is 0 25 For The Reaction A2 G B2 G C2 G D2 G If We Take 1 Mole Of Each Of The Four Gases In A
We can now substitute in our values for and to find.
. There will be very Ittle Ny and Oy Suppose a 90L reaction vessel isted with 13 mol of NO. At equilibrium the concentration of AB is 1825 M the concentration of BC is 1625 M and the concentration of AC is 0130 M. L reaction vessel is filled with 10 mol of NO and 10 mol of CO2.
SO2g NO2g SO3g NOg. Chemistry questions and answers. To solve this problem we can use the relationship between the two equilibrium constants.
At equilibrium the concentration of AB is 3225 M the concentration of BC is 2825 M and the concentration of AC is 0210 M. At a certain temperature the equilibrium constant for the chemical reaction shown is 160103. The chemical equilibrium constant means that at a certain temperature the reversible reaction starts from the forward reaction or the reverse reaction and regardless of the initial concentration of the reactants the equilibrium is finally reached.
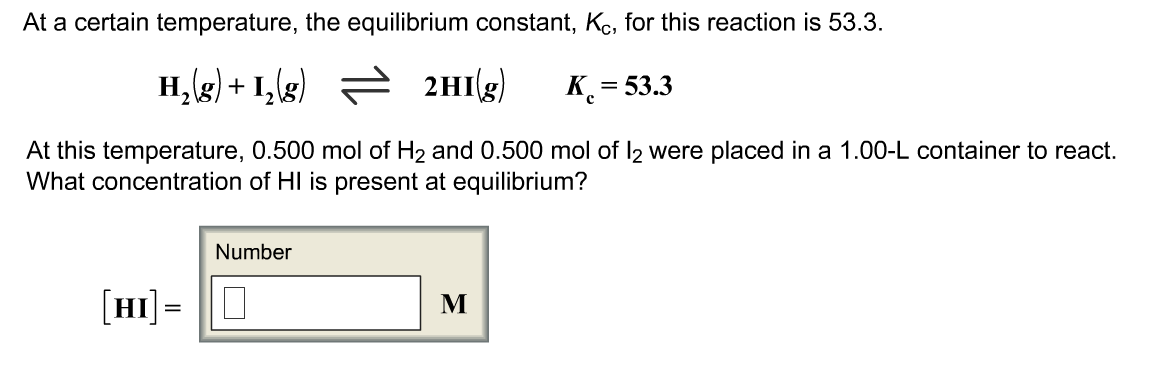
At a certain temperature the equilibrium constant for the following reaction is 00075. H2gI2g2HIgKc533 H2gI2g2HIgKc533 At this temperature 0400 mol H20400 mol H2 and 0400 mol I20400 mol I2 were placed in a 100 L container to react. At a certain temperature the equilibrium constant for the chemical reaction shown is 549 x 10-3.
SO2g NO2g arrow SO3g NOg At this temperature. R f r b Or kf α AaBb kb α Cc Dd. If 310 g Cl2 is placed in a 200 L flask at this temperature what are the equilibrium concentrations of Cl2 and Cl.
Chemistry questions and answers. The ratio of the rate constant of forward reaction to the rate constant of backward reaction should be a constant and is called an equilibrium constant K equ. At equilibrium the concentration of AB is 1925 M the concentration of BC is 2325 M and the concentration of AC is 0280 M.
At a certain temperature the equilibrium constant for the following chemical equation is 270. What concentration of HIHI is present at equilibrium. To find we compare the moles of gas from the product side of the reaction with the moles of gas on the reactant side.
At a certain temperature the equilibrium constant 𝐾c is 676103 for the reaction Cl2 g2Cl g A. At this temperature calculate the number of moles of NO2g that must be added to 264 mol of SO2g in order to form 110 mol of SO3g at equilibrium. Based on the StoPGoPS approach to problem solving.
0 m o l each of all the four gases is taken in a one litre container the concentration of N O 2 at equilibrium would be. At a certain temperature the equilibrium constant for the following reaction is 13. Predict whether the equilibrium constant for this reaction at 100C will be greater than less than or equal to 47 10-3.
At a certain temperature the equilibrium constant K c is 1 6 for the reaction S O 2 g N O 2 g S O 3 g N O g If 1. Use the gas constant that will give for partial pressure units of bar. SO2g NO2g SO3g NOg.
At a certain temperature the equilibrium constant Kc for this reaction is 533 H2 I2 2HI at this temperature 0800 mol of H2 AND 800. Calculate the concentration of B at equilibrium. At a certain temperature the equilibrium constant K for the following reaction is 849 x 101.
At a particular temperature the rate constants are constant. NCE 0 8 2NO Use this information to complete the following table. At a certain temperature equilibrium constant Kc is 16 for the reaction.
Calculate the concentration of B at equilibrium. At a certain temperature the equilibrium constant KcKc for this reaction is 533. At a certain temperature the equilibrium constant K for this reaction is 533.
At 25C the value of the equilibrium constant K c is 47 10-3. AB aqBC aqAC aq2B aq AB aq BC aq. At a certain temperature the equilibrium constant for the following chemical equation is 200.
Cl2 M Cl M Following the establishment of equilibrium in part A the volume of the flask is suddenly increased to. If the partial pressures of H2 and I2 were each 0300 bar initially what. H2gI2g-2HIg At this temperature the reactants were placed in a container to react.
At a certain temperature the equilibrium constant for the chemical reaction shown is 277103. NO g Cog - NOg Cog Use this information to complete the following table. NO g co s - NO g Co Use this information to complete the following table.
SO3g NO g NO2 g SO2 g was found to be 0500 at a certain temperature. L reaction vessel is filled with 17 mol of NO and 17 mol of Co.
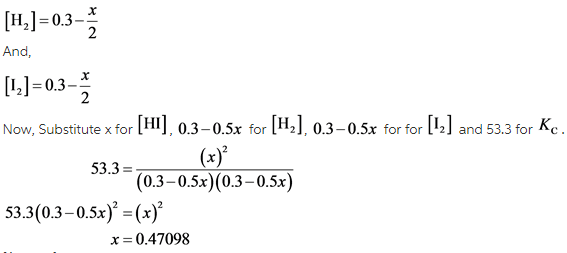
At A Certain Temperature The Equilibrium Constant Kc For This Reaction Is 53 3 Home Work Help Learn Cbse Forum
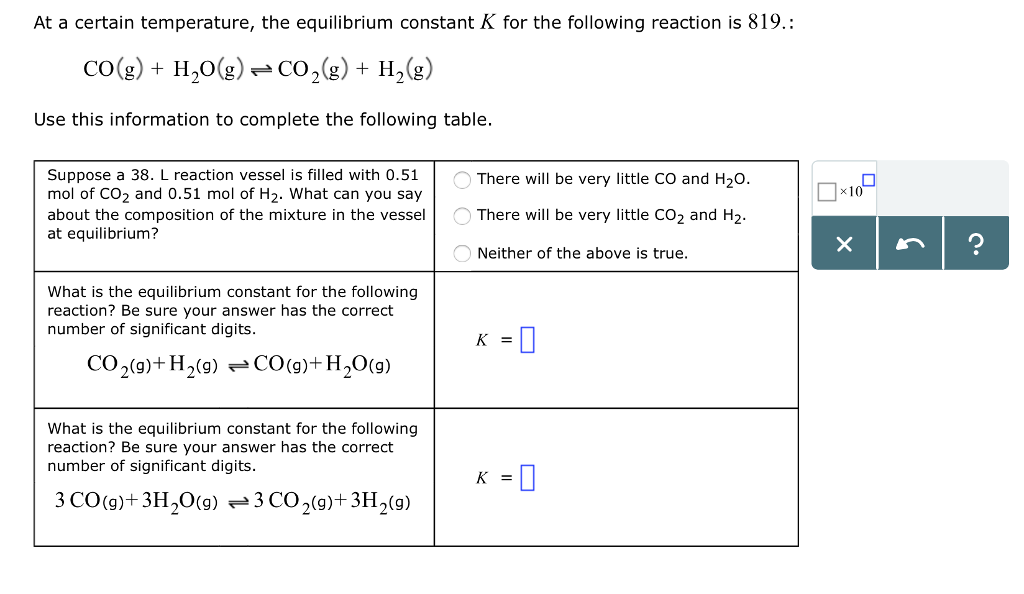
Solved At A Certain Temperature The Equilibrium Constant K Chegg Com

Solved At A Certain Temperature The Equilibrium Constant Chegg Com
No comments for "At a Certain Temperature the Equilibrium Constant"
Post a Comment